Non Invasive Monitoring - Task Force
New Task Force® cardio
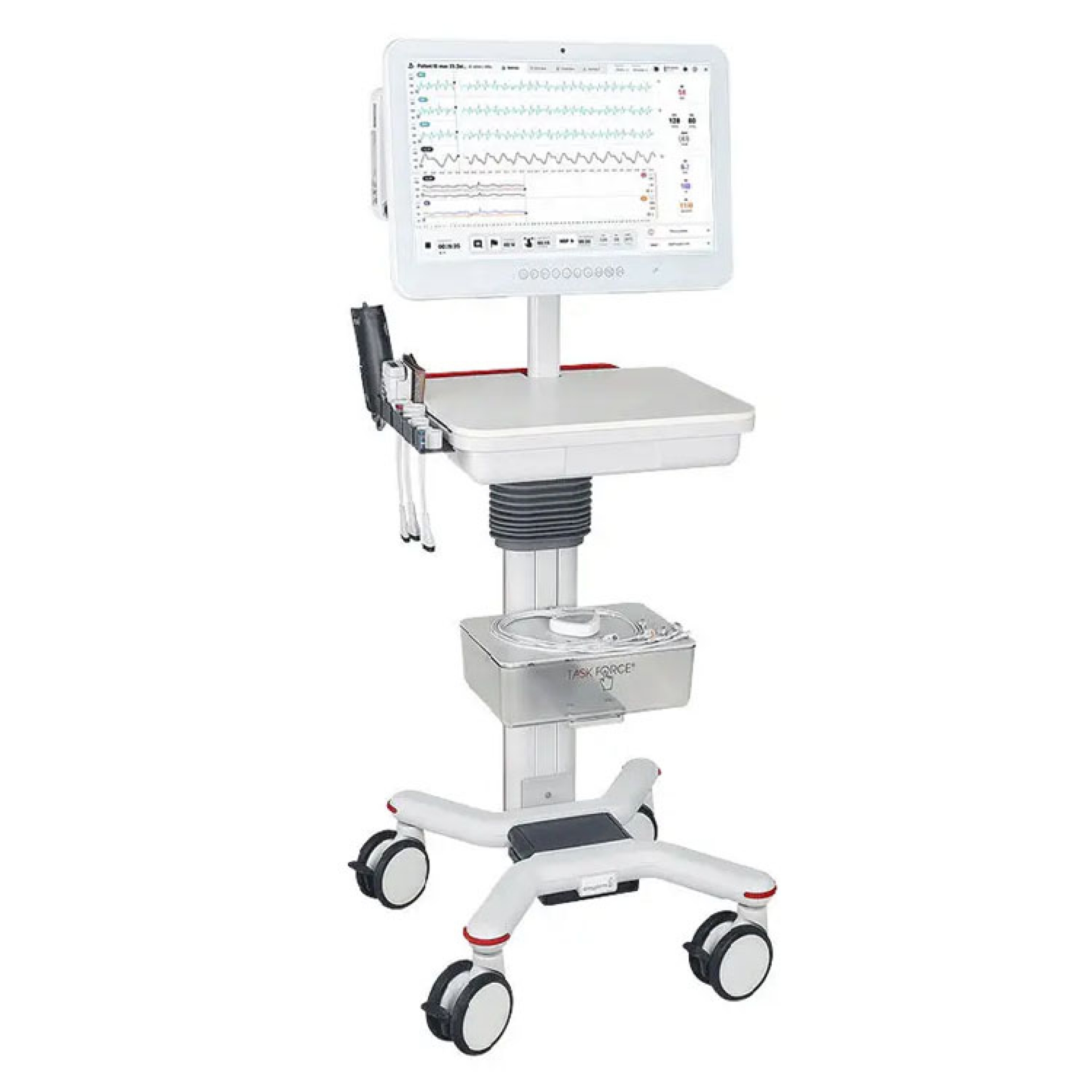
Advanced system for the detection and analysis of hemodynamic and electrophysiological parameters integrated in a single device.
Register and download the brochureRequest technical information
The system is able to simultaneously detect and analyze the following parameters:
CNAP® - Continuous non-invasive blood pressure: thanks to the double finger sensor the measurement is precise and reliable. The systolic, diastolic and average pressure arterial waveform,are detected in real time
Hemodynamic parameters - Continuous and non-invasive cardiac output with detection by the same finger sensor. It allows the monitoring of Cardiac Output, Stroke Volume and Peripheral Resistances in real time
NIBP - Brachial blood pressure with standard cuff: increases the accuracy level of the device
ECG - 12 high resolution leads with exclusive Bluetooth module that transmits the traces to the main unit without the interposition of cables.

Detected parameters
Beat-to-beat CNAP pressure
- Systolic, diastolic, mean
- Pulse pressure
- PP
- Waveform
Hemodynamic parameters
- Cardiac output, Stroke volume
- Peripheral resistors
NIBP pressure
12-lead ECG
Exclusive features
- Quick and easy patient preparation, thanks to the ergonomic accessories
- Display of traces and parameters in real time on a high resolution 24 '' monitor with Touchscreen technology to manage the entire exam with simple touches of the display.
- Ability to set customized examination protocols with automatic insertion of markers that identify the different phases.
- Input of comments and maneuvers on the patient
- Possibility, during the review phase of the examination, to identify portions of the path of interest, which can be inserted in the report and on which to calculate statistical data of interest.
- Advanced connectivity: LAN, Wi-Fi and Bluetooth
- System equipped with dedicated trolley, with adjustable height and housing for the various accessories.
- Natively certified according to the new MDR 745/2017
To download the data sheet, you need to access the restricted area:
Not registered?
Register nowWHAT ARE YOU LOOKING FOR?
-
Ultrasound Horus Wireless Ultrasound Probe Syncope Non Invasive Monitoring - Task Force Autonomic Nervous System Non Invasive Monitoring - Task Force Noninvasive Hemodynamic Monitoring Non Invasive Monitoring - Task Force Noninvasive Hemodynamic Monitoring - CNAP implantable Devices Optimization Non Invasive Monitoring - Task Force Noninvasive Hemodynamic Monitoring - CNAP
CLINICAL SPECIALTIES SEDA
CRITICAL CARE AND RESUSCITATION
CARDIOLOGY
GENERAL SURGERY
PLASTIC AND MAXILLOFACIAL SURGERY
VASCULAR SURGERY
DIALYSIS
INFUSION THERAPY
EMERGENCY
PHONIATRY
GASTROENTEROLOGY
NEUROSURGERY
NEURORADIOLOGY
ORTHOPEDICS, TRAUMA, HAND-FOOT SURGERY
ENT
INTERVENTIONAL RADIOLOGY
OPERATING ROOM ACCESSORIES
PAIN THERAPY
UROLOGY
REGISTER TO GET MORE TECHNICAL INFORMATION!
Register to get access to the complete and detailed descriptions of our products.
REGISTER NOW