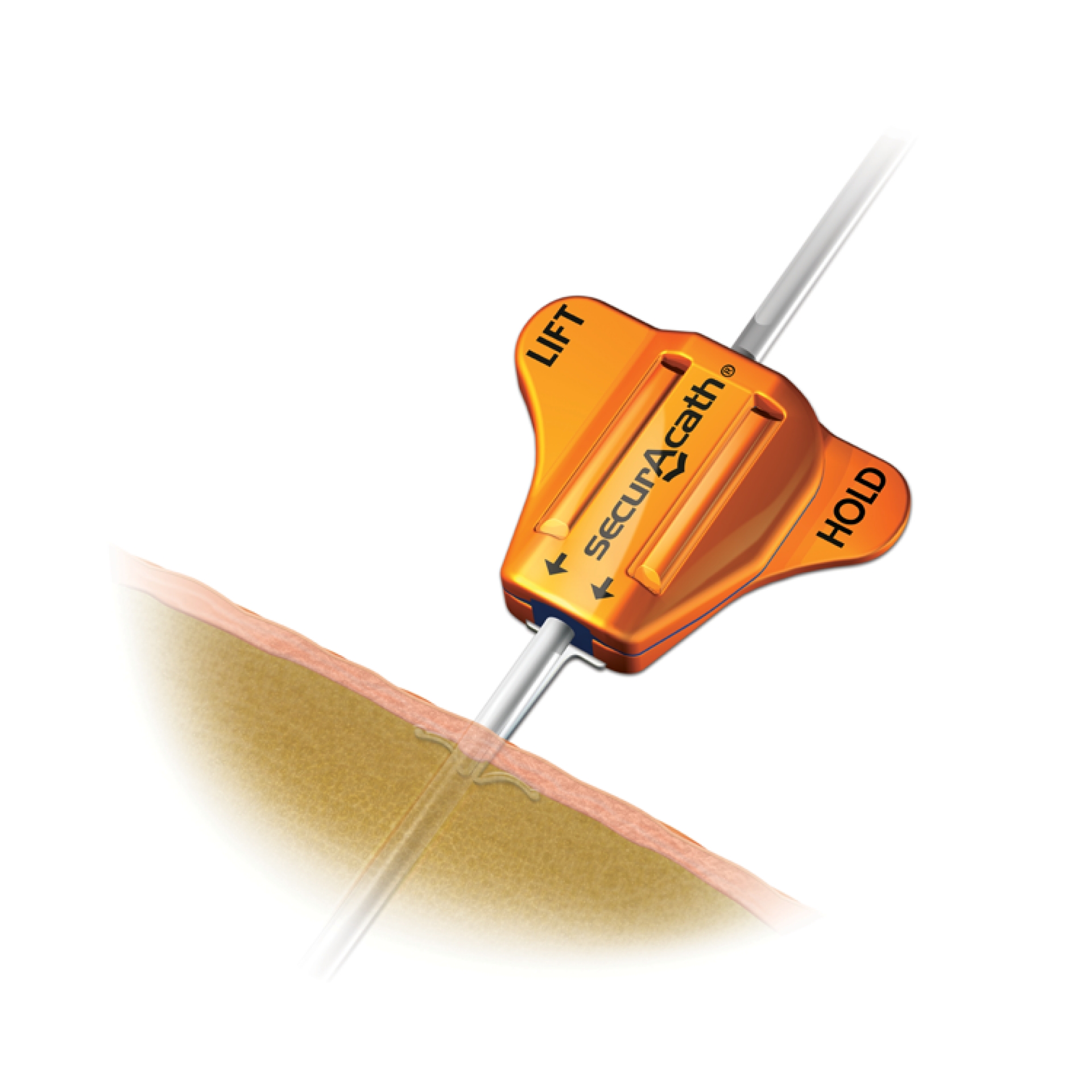
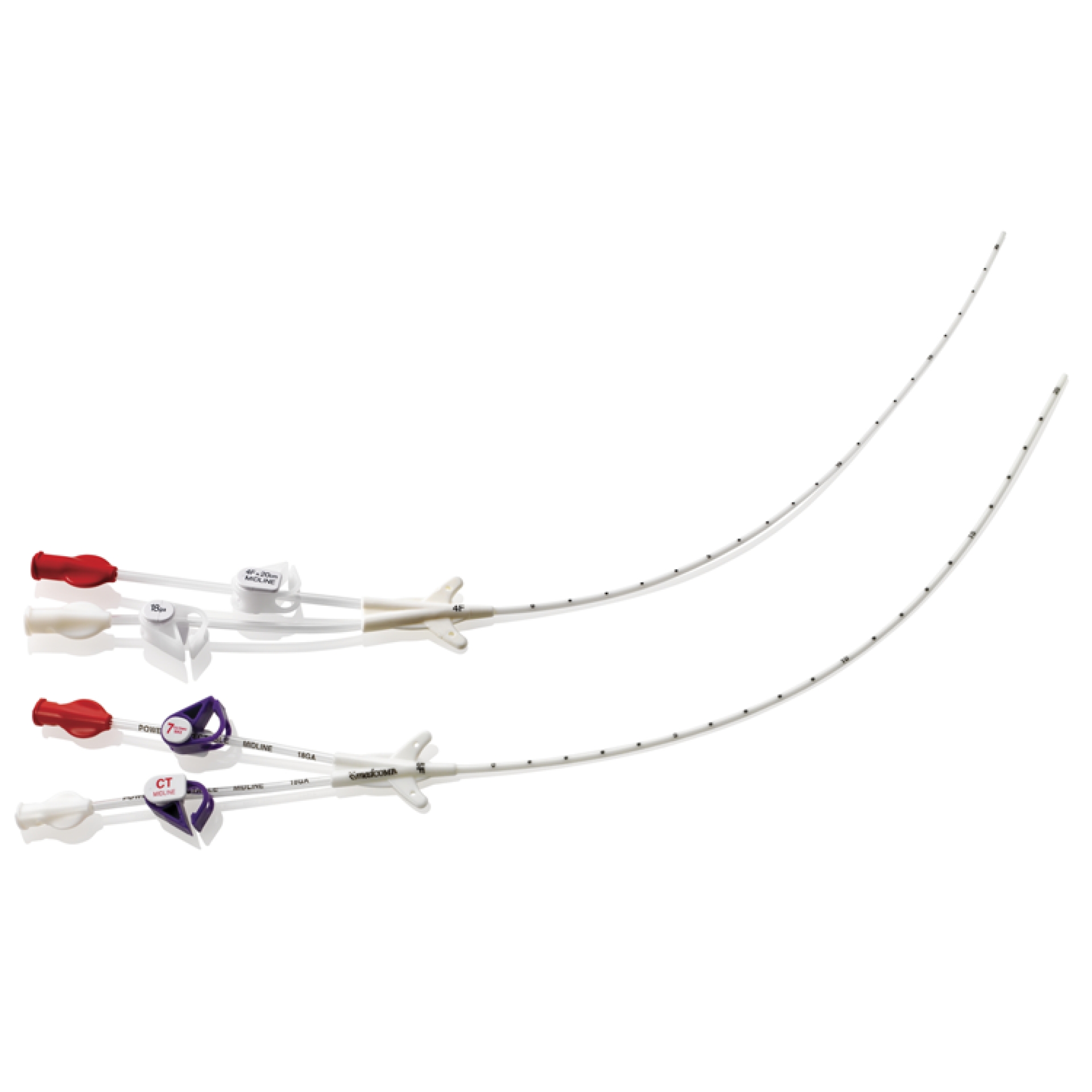
Subcutaneous Engineered Stabilization Device SecurAcath®
SecurAcath®, universal Subcutaneous Engineered Stabilization Device (ESD) for Catheter Securement
SecurAcath® device represents the New Standard of Care for life of the line. The system is universal and is compatible with any type of catheter (PICC line, CVC) and drainage from 3F to 8F.
Register and download the brochureRequest technical information
Advantages of SecurAcath®, subcutaneous securement midline device for catheter stabilization
SecurAcath® is the only Subcutaneous Engineered Stabilization Device (ESD) that meets the 2016 Infusion Therapy Standards of Practice*.
(*Journal of Infusion Nursing, Vol. 39, No. 1S, Jan/Feb 2016)
- SecurAcath® device prevents therapy interruption
- SecurAcath® device may improve vessel health and preservation
- SecurAcath® device lowers total cost of patient care
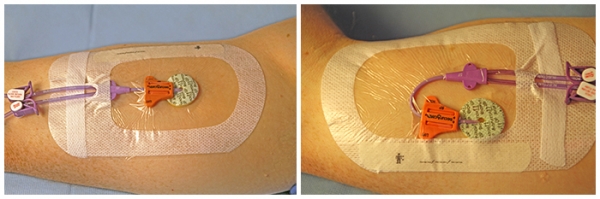
SecurAcath® device benefits: improved efficiency
- One SecurAcath® device secures for the life of the line
- Catheter remains secure during dressing changes
- Saves time during routine dressing change - Dressing change can be done 3–5 minutes faster
- Allows easy catheter repositioning if catheter tip must be pulled back
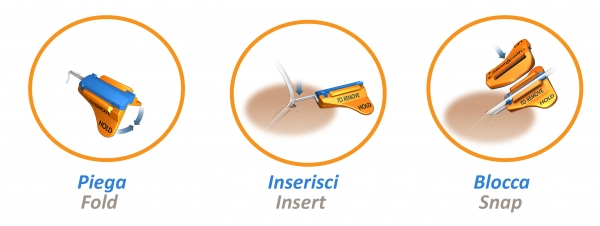

SecurAcath® device benefits: 360 Degree Site Cleaning While Secured
- Excellent cleaning access around the entire insertion site
- Catheter remains stable and secure during cleaning
- Improved stability and cleaning may help reduce infections

SecurAcath® device benefits: dramatically Reduced Catheter Dislodgement
- The use of SecurAcath® Catheter Stabilization Device showed a drastic reduction of catheter dislodgement rates with respect to adhesive systems
- Many accidental dislodgements occur during dressing changes when catheter is not secured
- Decreased catheter replacement costs

To download the data sheet, you need to access the restricted area:
Not registered?
Register now1. What is SecurAcath® used for?
SecurAcath® is a midline securement device that stabilizes catheters subcutaneously, ensuring safe and continuous securement for the entire dwell time.
2. Which catheters is it compatible with?
It is compatible with various catheter types and sizes; refer to the technical documentation for compatibility with product codes.
3. How long can it remain in place?
SecurAcath® is designed to remain in place for the entire dwell time, maintaining continuous catheter securement without the need for replacement at dressing changes.
WHAT ARE YOU LOOKING FOR?
-
Tip Location and Ultrasound Systems for Vascular Access Albatros Wireless Tip Location with Linear Convex Cardio Ultrasound Probe Magellano Intracavitary ECG ECG leads for Tip Location Subcutaneous Engineered Stabilization Device SecurAcath® Subcutaneous Engineered Stabilization Device SecurAcath® A better way to manage lines connections Yewtwist Infusion accessories EpiFaith ® CV Accessories Catheters Locking Solutions DURALOCK-C Hemostatic devices GuardaCare® OneStop™ Vascular Dialysis Catheters Long Term Catheters Short Term Catheters Peritoneal Cathters Pediatric Hemodialysis Cathters PICC and Midline Catheters PICC Catheters Cuffed PICC Catheters Midline Catheters Port CVC Micro-Introducer Set Micro-Introducing Set
CLINICAL SPECIALTIES SEDA
CRITICAL CARE AND RESUSCITATION
CARDIOLOGY
INTERVENTIONAL CARDIOLOGY
GENERAL SURGERY
PLASTIC AND MAXILLOFACIAL SURGERY
VASCULAR SURGERY
DIALYSIS
INFUSION THERAPY
EMERGENCY
PHONIATRY
GASTROENTEROLOGY
NEUROSURGERY
NEURORADIOLOGY
ORTHOPEDICS, TRAUMA, HAND-FOOT SURGERY
ENT
INTERVENTIONAL RADIOLOGY
OPERATING ROOM ACCESSORIES
PAIN THERAPY
UROLOGY
REGISTER TO GET MORE TECHNICAL INFORMATION!
Register to get access to the complete and detailed descriptions of our products.
REGISTER NOW